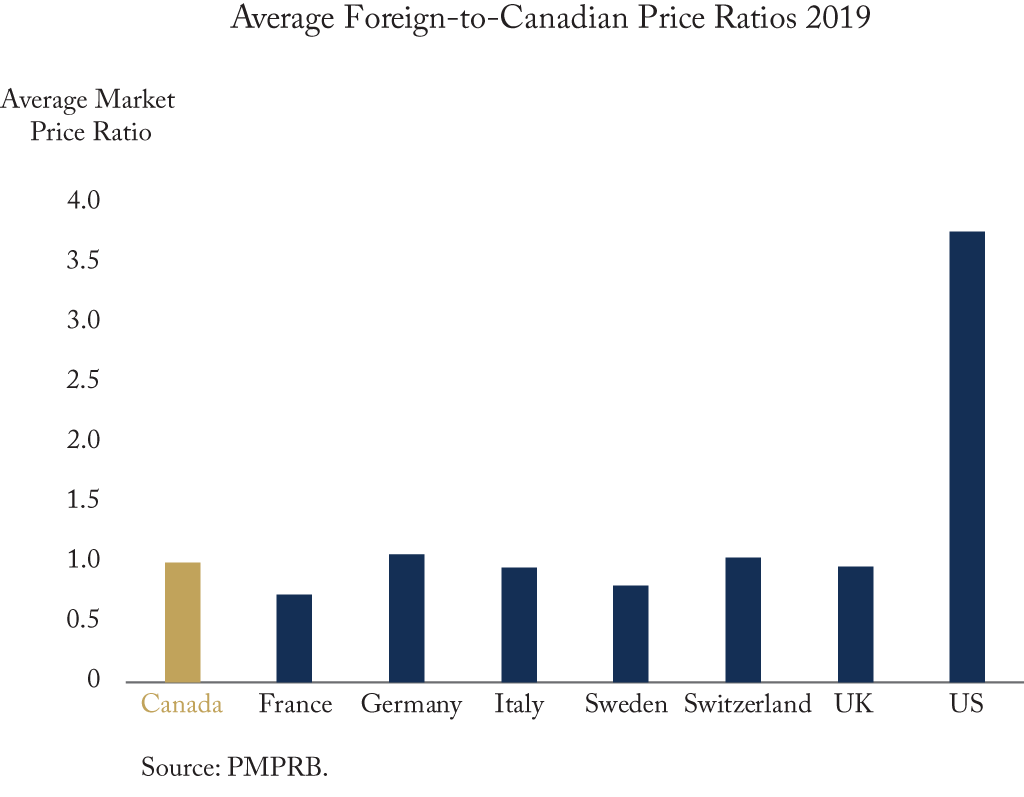
Canadian drug prices are lower than in the U.S. but higher than in many other peer countries. The federal government is planning to introduce new patented drug pricing rules in January 2022; but is this the right solution?
In pursuit of affordable healthcare, via lower drug prices, we must keep in mind the need to discover and develop new and more effective drug therapies with more R&D. As a high-income industrialized country, Canada is at risk of falling short of its expected contribution to global R&D financing, if it pursues a policy of strong price regulation.
Instead of implementing stricter price regulation, we propose the new Canadian Drug Agency (CDA) be empowered to achieve lower prices through negotiation with manufacturers, on behalf of private and public plans. The role of the Patented Medicine Prices Review Board (PMPRB) should be redefined to focus on collecting and reporting data on Canada’s contribution to global R&D financing.
On a parallel track, Canada should support the efforts of multilateral institutions such as the World Health Organization and others to create a less contentious international burden sharing system.
To learn more about our Canadian drug-pricing policy recommendations, read “Cutting Square Deals: Drug Prices, Regulation, and Patent Protection”, by Åke Blomqvist and Paul Grootendorst.