From: Rosalie Wyonch
To: Canadians Concerned about Healthcare
Date: April 13, 2021
Subject: Drug Shortages and Domestic Manufacturing
Lost in Canada’s lamentations about vaccine shortages and domestic manufacturing of medicines is the bigger picture of drug shortages in Canada.
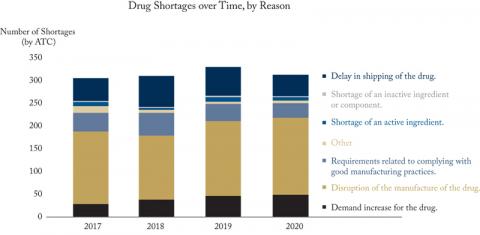
In 2020, there were 4,980 new drug shortages reported (excluding “anticipated shortages”) in Canada covering 313 Anatomical Therapeutic Chemical (ATC) classifications, the internationally comparable classification codes. As of March 15, more than 90 percent of those shortages have been resolved or avoided. While thousands of drug shortages in a year might sound like a crisis, there were similar numbers in 2017, 2018 and 2019 for an average of 5,684 shortages over 315 ATCs.
Similarly, reasons for drug shortages at the manufacturing level show little change from previous years. In 2020, 54 percent of shortages were due to disruptions in manufacturing and 2.2 percent were due to a shortage of active ingredients: both active concerns as international and domestic policies put in place to mitigate the pandemic caused significant disruption to the production of goods and moving them across borders. In 2019, 50 percent of shortages were due to manufacturing disruptions and 2.9 percent were due to shortages of active ingredients (see Figure).
A similar number of drug shortages in crisis and non-crisis years suggests they are not a rare emergency situation, but rather a chronic and growing concern in Canada’s healthcare system. Surveys of pharmacists, physicians and specialists have documented the extent of drug shortages affecting the majority of practitioners in every province, and find them present over a wide array of different products. The problem is not unique to Canada. In the US, with its large drug manufacturing base, there were still more than 100 drugs in active shortage prior to the pandemic, contributing an additional $230 million in annual drug costs nationwide, measured by wholesale price fluctuations pre- and post-shortages.
There are many reasons that drug shortages arise: disease outbreak, manufacturing quality issues, natural disasters and difficulty sourcing raw ingredients, for example. Similarly, drug shortages could arise at the national, provincial, regional or facility level with possible causes rippling through the supply chain: distribution delays, lack of appropriate infrastructure to store medicines, or even administrative errors in ordering can all contribute to shortages. A US Food and Drug Administration report identifies three root causes for drug shortages: lack of incentives for manufacturers to produce less profitable drugs; no market incentives for “mature quality systems” that focus on continuous improvement and early detection of supply chain issues; logistical and regulatory challenges that make recovery from disruption difficult.
Many researchers, healthcare providers and industry groups and associations have illuminated various aspects of the drug shortage problem and recommended ways to mitigate and manage them in Canada. Though Canada has had mandatory reporting of anticipated and actual drug shortages since 2016, the system lags the US where the FDA has dedicated staff responsible for the coordination of drug shortage prevention and mitigation activities, the monitoring of ongoing shortages and monitoring the supply of products with recently resolved shortages.
The pandemic and recent focus on vaccines have made the issue of consistent access to essential medicines an even higher priority than it was before. The complex and varied causes of drug shortages, however, existed prior to the pandemic, as did the shortages. Addressing drug shortages in Canada will require more than government investment in manufacturing. It will require addressing the complex causes arising all along the supply chain from macro-market dynamics to regional challenges at the care-delivery level.
Rosalie Wyonch is a Senior Policy Analyst at the C.D. Howe Institute.
To send a comment or leave feedback, email us at blog@cdhowe.org.
The views expressed here are those of the author. The C.D. Howe Institute does not take corporate positions on policy matters.
Source: Drugshortages.ca, author’s calculations
Notes: Shortage data is grouped by ATC codes to account for multiple shortage reports arising from similar medications in different strengths/dosages. Year is determined by the “actual start date” of shortages reported to drugshortages.ca. If no “actual start date” is available, assumed start date is the date of the initial report. “Anticipated shortages” have been removed.