The Study In Brief
The frequency of viral outbreaks and pandemics in the last two decades, including the current COVID pandemic, has focussed attention on Canada’s pandemic preparedness generally and its pandemic vaccine development and production self-sufficiency, in particular. As Canada faced disruptions in the supply of COVID vaccines from other countries, there were numerous calls for a public agency that would be charged with pandemic vaccine production and possibly vaccine development as well.
In this paper, we review the prospects for such an agency and note that it would be risky to rely on it to rapidly develop and test a vaccine, given the high failure rates for vaccine development projects and logistical challenges in mounting a large-scale clinical trial in short order. But a public agency that instead focussed on producing vaccines licensed from domestic or international developers would also face challenges. The primary issue is one of production readiness. Practically, production facilities need to be operating continuously at or near full-scale capacity to hone the processes needed to meet stringent and evolving regulatory standards and ensure personnel have sufficient experience. Facilities also need a reliable supply of key inputs. The public agency would thus need to be engaged in full-scale vaccine production even in non-pandemic times to maintain both production know-how and stable input supply chains. It would need to do so in three production platforms that may be needed to produce vaccines for the next pandemic virus. This raises the question of what vaccines the agency would routinely produce, in non-pandemic times, and where the vaccines would be distributed.
Our view is that Canada can achieve a more reliable supply of vaccines for future pandemics, and at lower cost, by contracting with existing commercial producers that are already engaged in continuous and full-scale production and who thus have demonstrated technical competency and have secure input supply chains. The government can purchase either reserve capacity in existing domestic commercial production facilities or can cover the cost of an adjacent modular production facility that can thus tap into the steam, gas and other utilities needed to run the facility. The number and capacity of the vaccine manufacturing platforms that Canada requires depends on whether Canada can negotiate an agreement with other countries that allows each country to specialize in a platform and share pandemic vaccines with partners should the need arise. Regardless of its approach, however, Canada needs to act soon if we are to be ready for the next pandemic.
Introduction
Viral epidemics and pandemics are occurring with increasing frequency. There were four major epidemics and pandemics between 1918 and 2000: the 1918-1920 “Spanish flu,” the 1958-59 “Asian flu,” the 1968-69 “Hong Kong flu,” and HIV/AIDS pandemic.
There have already been six major viral outbreaks since 2000: the 2002-04 severe acute respiratory syndrome (SARS) outbreak, the 2009 “H1N1 flu,” the 2012 Middle East respiratory syndrome (MERS) outbreak, the 2014 Ebola outbreak in West Africa, 2015-2016 Zika outbreak, and the 2019-21 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic (Council on Foreign Relations n.d.).
These post-2000 outbreaks were caused by zoonotic viruses; i.e., viruses that originated in animals and spread to humans, often through an intermediate animal host. This animal-to-human viral transmission appears to be caused by the mixing of animal species (such as pigs and ducks) in farms and live animal markets. (Wolfe et al. 2007; Xiao et al. 2021) The subsequent human-to-human viral transmission is facilitated by high levels of intra- and international travel and migration (Vignier and Bouchaud 2018).
The distribution of costs – across the outbreaks – is right skewed, with most outbreaks imposing modest costs, but a few imposing extremely large costs. For example, the 2009 H1N1 flu outbreak killed about 284,000 globally (Dawood et al. 2012); this death toll, while sobering, is similar to the annual global mortality from seasonal influenza (Paget et al. 2019). By contrast, the SARS-CoV-2 pandemic, or more accurately the respiratory illness, coronavirus disease 2019 (COVID-19 and hereafter, just COVID), which is caused by the SARS-CoV-2 virus, has killed about 20 times as many, at least according to the official statistics. As of mid-February 2022, the pandemic is estimated to have caused about 20 million “excess deaths” – deaths during each month of the pandemic, which are over and above what was typical each month prior to the pandemic (Adam 2022). Moreover, many of those who recover from severe COVID will suffer long-term impairments (Cutler and Summers 2020; Canadian Press 2021).
Pandemics also impose costs other than those that result directly from infection. The lockdowns and related measures enacted to reduce contagion have reduced incomes; the income losses from the disruption in trade during the COVID pandemic are measured in the trillions of dollars (Gopinath 2020; Rynne et al. 2020; The World Bank 2020). The social isolation arising from lockdowns also takes a toll on mental health (Abbott 2021).
Unfortunately, future viral pandemics seem likely. An estimated 631,000 to 827,000 mammalian and waterfowl viruses, from 25 different viral families, have zoonotic potential (Carroll et al. 2018). Viral outbreaks can also be accompanied by opportunistic fungal or bacterial infections against which current antimicrobials are infective (Bhatt et al. 2021). Finally, we cannot completely discount the possibility of the release into the environment of genetically engineered pathogens which combine epidemic or pandemic potential with highly challenging vaccine development characteristics (MacIntyre and Bui 2017). Ensuring that Canada has priority access to vaccines for future pandemics has thus become a pressing public policy concern. But how should Canada enhance its defence against infectious threats?
One option is to purchase finished dosage-form pandemic vaccines on the world market – as we have for the COVID pandemic. However, there is a risk of encountering supply disruptions caused by either manufacturing problems in foreign plants, or so-called “vaccine nationalism”: export restrictions imposed by governments in vaccine-producing countries. Indeed, shipments of the Pfizer and Moderna vaccines to Canada were delayed due to production problems (Loftus and Vieira 2021; Tasker 2021a, 2021b). Also, the US and Indian governments imposed vaccine export bans that delayed shipments of the Pfizer and AstraZeneca vaccines to Canada (Lexchin 2020; Tumilty 2021; Wingrove 2021).
Given the risk of future supply disruptions, a case can be made to expand Canada’s domestic vaccine capacity. To this end, various academics, (Deveaux 2021; Herder and Murthy 2021; Rutty 2020) politicians (NDP 2020; Reynolds 2021) and other commentators (Barris 2021; Barry 2021; Campbell 2020; Darrah 2020; Editorial, The Times Colonist 2021; McQuaig 2020a, 2020b) have proposed that Canada create a public agency devoted to pandemic vaccine development and production. Advocates suggest that this approach is feasible given that Canada once had two successful publicly owned vaccine producers.
We agree that Canada needs a plan to have a vaccine available to immunize the population as quickly as possible and contribute to the world supply following the onset of the next pandemic. The adage that “no one is safe until everyone is safe” has been shown to be true during the current COVID pandemic. Given the significant wider benefits and public good aspects of vaccine use and financing, relying on individual consumer demand will result in underinvestment. Thus, public funding is needed to expand Canada’s domestic vaccine production capacity. We also agree that Canada needs a federal agency that can plan for and respond to pandemics.
However, our view is that relying on a new public agency to develop and produce vaccines for the next pandemic is ill advised. We argue that Canada can achieve a more reliable supply of vaccines for future pandemics, and at lower cost, by contracting with existing commercial producers that are already engaged in continuous and full-scale production, and who thus have demonstrated they have technical competency and secure input supply chains. The government can purchase either reserve capacity in existing domestic production facilities or can cover the cost of a modular production facility on the grounds of an existing production facility and can thus tap into the ventilation, steam, gas and other utilities needed to run the facility. Canada can also enter into agreements with other countries (like Australia and the UK) to rationalize production and share pandemic vaccines.
This paper proceeds as follows. First, we briefly describe the history of Canada’s publicly owned vaccine producers, Connaught Labs and Institut Armand Frappier. Next, we outline the challenges that a new public agency – a “Connaught 2.0” – would face in each stage of vaccine development and production. Finally, we discuss how the federal government can leverage Canada’s existing capacity in each of these stages to enhance pandemic vaccine supply security.
| Key Concept Explainer |
|---|
|
Vaccine Platforms Vaccines are produced using “platforms” that can be grouped into four categories. Each method presents (either directly or indirectly) the immune system with an antibody generator or “antigen” that primes the immune system to neutralize the virus among those who subsequently become exposed. Whole Virus (live attenuated, inactivated): The first and oldest method is to grow whole viruses, in fertilized chicken eggs or other live animal cell cultures in large-scale fermenters. The vaccine viruses are weakened or attenuated so they cannot produce disease but still cause the body to mount an immune response against the virus. Alternatively, the whole viruses are inactivated, using either heat or a chemical treatment, rendering them unable to infect and replicate. The remaining vaccine production platforms use lab created, or “recombinant” DNA. DNA sequences, or genes, contain the code that instructs cells to make one or more proteins. Protein (protein subunit, virus-like particle): The protein-based platforms deliver this instruction set into a cell that is cultured in a lab; this could be an animal, insect, plant or microbial cell. Once inside this cell, the DNA is converted within the cell’s cytoplasm into RNA, which instructs the cell to produce the small but important pieces (subunits) of the virus envelope that the human immune system can recognize and respond to. For SARS‐CoV‐2, the spike protein, which sticks out of the virus envelope, is commonly used. These cell-made virus proteins, including the spike protein, can also be expressed on the outside of a particle that is about the size of a virus. As it also has the shape and size of a virus, the expectation is that the protein delivered on a virus-like particle would induce an even broader response by the body's immune cells. Other vaccine platforms do not contain the inactivated virus or virus proteins but instead contain the genetic code for the viral proteins. This vaccine, once injected, delivers this genetic code to the inoculated person’s own cells, which in turn produce and introduce the protein of interest to the immune system. In essence, the person’s own cells are making the vaccine. Nucleic Acid (DNA, RNA): If DNA is introduced into the cells of the inoculated person, the DNA moves to the cell nucleus where the code is transcribed to make mRNA (m for messenger) that takes the message to the body of the cell where it acts as the template to make the protein of interest, such as the SARS‐CoV‐2 spike protein. RNA vaccines skip the DNA step, and transport mRNA directly into a person’s cells. The mRNA directly codes the production by the host cell of the protein of interest that will induce an immune response against the virus. Viral Vector (non-replicating, replicating): The viral vector vaccine platforms get the DNA code into human cells using a non-pathogenic virus shell to carry and “infect” the inoculated person’s cells. The most common vector is an adenovirus (one of many common cold viruses). The virus shells that transport the DNA can be ones that do not replicate in human cells. For a fuller discussion, see the Online Appendix. |
Canada’s Publicly owned Vaccine Producers: A Short History
Canada’s history of vaccine R&D and manufacturing begins with the Connaught Laboratories. Originally known as the Antitoxin Laboratory, Connaught Labs was founded in 1914 through the donation of a farm just north of Toronto by Col. Albert Gooderham, Chair of the Ontario Red Cross and owner of Gooderham Distilleries, to the University of Toronto, Department of Hygiene. The donor required that the facility be used for the mass production of tetanus toxoid for the troops during WWI and that it be named after the Governor General of Canada, the Duke of Connaught (Last et al. 1991). The first Director was John FitzGerald, a physician and public health scientist. The foundations of public health and vaccine research, development and manufacturing were thus linked in Canada and remained intertwined for the next half-century (Defries 1968).
Over the years, Connaught made advances in the development and manufacture of vaccines for diphtheria, pertussis, tetanus, smallpox and polio, among others. Toronto was the first jurisdiction in the world to eradicate diphtheria thanks to the diphtheria toxoid vaccine Connaught developed in the early 1930s. The company went on to play a leading role in World Health Organization (WHO) efforts to eradicate smallpox and was one of only two reference laboratories established by the WHO to set international standards for smallpox vaccine production (Barreto and Rutty 2002). Connaught also played a significant role in its collaboration with Jonas Salk in the development of the polio vaccine and ultimately was a leading contributor to the eradication of polio through the supply of hundreds of millions of doses of Sabin vaccine to countries around the globe (Rutty et al. 2005). Connaught Labs was also the site of non-vaccine medical research. Most famously, Frederick Banting and Charles Best used the facility to develop insulin (Rutty, n.d.-a).
The federal government acquired Connaught Labs in 1972 from the University of Toronto; Connaught became a wholly owned subsidiary of the Canada Development Corporation (CDC). The CDC was a public-private partnership established to preserve Canadian ownership of certain “critical industries” (Rutty n.d.-b). It was a for-profit organization that operated at arm’s length from the government. Although both government and individual Canadians had equity stakes, the government equity share was intended to decline over time and be no more than 10 percent.
During the period from 1972 to 1990, Connaught made the transformation from a university-based, cost-plus R&D and manufacturing facility to a profit-oriented international company exporting vaccines to the US and elsewhere. In 1978, Connaught acquired a US subsidiary through the purchase of the Salk Institute/Merrell National facility in Swiftwater, Pennsylvania making Connaught the largest vaccine manufacturer in North America. It was also engaged in plasma fractionation, and the production of insulin, veterinary vaccines, and human diagnostics.
Connaught Labs, however, eventually faced headwinds. Although Connaught was a commercial enterprise, it evolved from an organization that had a strong academic and philanthropic culture. Most of its management positions were held by former academic scientists. As a result, the company was top heavy with brilliant scientists able to turn new ideas into new products but underrepresented by people with the business acumen needed to ensure its long-run survival. Thus, R&D was not sufficiently focused on those projects that would repay – on an expected basis – their sunk R&D costs with revenues earned on the sale of these new products. Moreover, it was struggling to launch new products that would keep pace with those launched by its competitors. This was particularly the case in its insulin and veterinary vaccine divisions where Connaught was losing market share to US firms. Meanwhile, plasma fractionation costs and liability exposure soared in 1984 due to concerns that its donor blood supply had been contaminated by the newly emerging HIV virus. The result was that during the 1980s, Connaught was losing money in three of its divisions and ultimately shut them down.
This allowed Connaught to focus on its vaccine division – which had been reliably profitable – but sales revenues declined as prices paid for its pediatric vaccines, the core of its vaccine portfolio, declined. These price declines occurred in part on account of the procurement policies of UNICEF and PAHO, agencies of the WHO that purchased vaccines for consumers in lower- and middle-income markets. These agencies used a winner-take-all tendering system, awarding all sales to the lowest bidder. But Connaught was also facing pricing pressure in the US market. The Centers for Disease Control and Prevention (CDC), a major purchaser of pediatric vaccines, also used a tendering system and the CDC’s US market share was increasing. As Danzon and Pereira (2011) note, during the 1980s the CDC purchased around 30-40 percent of vaccines for diphtheria, tetanus and pertussis (DTP) and polio in the US, and about 40-50 percent for measles, mumps and rubella (MMR). This resulted in low prices and great volume uncertainty for Connaught and other suppliers. And although Connaught remained the leading seller of pediatric vaccines in North America, it was losing its critical meningitis vaccines sales to a cheaper US product.
Connaught was also incurring additional costs to meet increasingly stringent safety and quality control standards in its vaccine manufacturing facilities. Despite the heightened safety standards, manufacturers selling in the US grew increasingly concerned over vaccine-injury liability exposure (Holland 2017). The decline in vaccine sales revenues coupled with the growth in its operating costs threatened Connaught’s one remaining profit centre. To reverse its fortunes, Connaught considered expanding the scale of its vaccine operations and pursuing more focused R&D. However, it had insufficient retained earnings to finance these initiatives and was unsuccessful in raising funds in capital markets. A Maclean’s article at the time stated:
For Connaught chairman Brian King, who has been trying to find a partner for the company since 1987, there was no doubt that the sale was necessary. Said King: “Heritage is great stuff, but it does not put meals on the table for employees.”(Walmsley 1989.)
The Canada Development Corporation put Connaught up for sale in 1986, attracting the attention of Institut Mérieux in France. Mérieux wanted the two Connaught manufacturing assets in Toronto and Swiftwater as a means of entering the North American vaccine market, while expanding its vaccine portfolio and manufacturing capacity. Canada agreed to the sale of Connaught in 1989, subject to various conditions. The full list of conditions was not disclosed but some were made public. These included a commitment to earmark 25 percent of Connaught’s spending to advanced biotechnologies and guarantees of Canadian priority access to all vaccines made by Connaught in the event of global shortage. A Canadian Board, independent of Mérieux, was established to ensure the fulfilment of the conditions (Walmsley 1989). The sale made Pasteur-Mérieux-Connaught the largest vaccine manufacturer in the world at the time (Sanofi Pasteur 2021).
Mergers and acquisitions during the 1990s led to continued growth and investment at both the Connaught (Toronto) and Swiftwater sites, with several name changes along the way (Mayer 2020). The original Connaught site is still outside of Toronto, now bearing the name Sanofi, one of the world’s largest vaccine manufacturers.
Institut Armand Frappier, or IAF, also has academic origins. It was founded in 1938 as Institut de microbiologie et d’hygiène de l’Université de Montréal by Armand Frappier, a noted physician and microbiologist. The Institut produced, among other things, Bacillus Calmette–Guérin (BCG - a vaccine against tuberculosis), DPT, polio and influenza vaccines for both domestic and international use (Payment 2014). Like Connaught, IAF began to face financial headwinds in the 1980s. Its retained earnings were insufficient to fund the R&D projects needed to keep up with the product innovations of its competitors. For instance, adjuvanted DPT combinations were introduced by Connaught in 1980 and IAF was unable to develop an equivalent product. Its non-adjuvanted product was grandfathered by Health Canada to supply Quebec but it had limited sales in the rest of Canada. In 1989, IAF’s commercial vaccine arm was sold to Biochem Pharma and a new influenza vaccine manufacturing facility was built at Ste. Foy Quebec in 1998 under the subsidiary named BioChem Vaccines (Payment 2014). The company was bought by Shire Pharmaceuticals in 2001 and the influenza manufacturing and R&D facility was ultimately sold to GlaxoSmithKline (GSK). The facility produces the majority of Canada’s seasonal influenza vaccine and also exports seasonal influenza vaccine to the US. In addition, GSK entered into two ten-year contracts with the government of Canada as its pandemic influenza vaccine supplier. During the 2009 H1N1 pandemic, the GSK facility produced 30 million doses of pandemic influenza vaccine for Canada. The pandemic contract expired in 2021.
One might question whether Connaught Labs and IAF would have prospered and remained Canadian-owned if they simply had been managed better. There are reasons to suggest that the answer is no. Connaught Labs and IAF were just two of the many vaccine manufacturers that merged with, or were acquired by, other manufacturers during the period starting in the late 1980s and ending in the early 2000s. Lederle Laboratories was a major competitor to Connaught in the US market during the early 1990s and was owned by American firm Cyanamid. It was bought by Wyeth, which was acquired by Pfizer in 2009. Welcome Laboratories, a UK vaccine company, was bought by Glaxo in 1995 and GlaxoWelcome merged with SmithKline Beecham in 2000 to become GSK. In 2018, GSK bought the vaccine division of Novartis – except for its influenza vaccines, which were purchased by Commonwealth Serum Laboratories (CSL) in 2014, and was subsequently renamed “Seqirus” in 2015. IAF Biovac was purchased by Shire in 2001 and subsequently by GSK. AstraZeneca entered the vaccine business through the acquisition of Medimmune in 2007.
As was noted earlier, the consolidation in the vaccine industry during this time was caused by higher production costs from heightened regulatory safety standards; concerns over product liability may have also caused some firms to exit. Remaining firms grew large to amortize these growing overhead costs over a larger production volume (Institute of Medicine (US) 2003) and also replace the production capacity of the firms that exited the industry. The winner-take-all (or winner-take-most) vaccine procurement strategies of the CDC, the Pan American Health Organization (PAHO), UNICEF and other public vaccine procurement agencies reinforced this trend. Only a few vaccine producers can cover the cost of building and/or maintaining productive capacity in a market where large supply contracts are awarded to just one or two firms. The result is that about 80 percent of global vaccine sales revenues prior to the COVID pandemic were earned by just four large multinational corporations: GSK, Sanofi, Merck, and Pfizer. Thus, the odds were certainly stacked against Connaught Labs and IAF; it would have been difficult for them to remain independent, self-sustaining businesses given the transformation of the industry at the time.
The Prospects for a Connaught 2.0 Model of Vaccine Development and Production
Given that, at one time, Canada had two viable publicly owned vaccine producers, it is reasonable to ask if a revamped public agency – a Connaught 2.0 – would be feasible and desirable. To do so, however, it is helpful to first define the mandate of the proposed agency. Proponents of a Connaught 2.0 clearly want a public agency capable of producing pandemic vaccines domestically, within months after the start of a pandemic. It seems less likely that its proponents want an agency that is also capable of developing vaccines domestically. In a crisis, the only reasonable strategy would be to license the production of any vaccine that is effective, wherever it has been developed. Nevertheless, it is instructive to review what self-sufficiency in both the development and manufacture of pandemic vaccines would entail.
The development pathway of a new vaccine consists of eight steps: 1) research and development (R&D) culminating in a vaccine candidate that is then tested on animals; 2) production of modest quantities of vaccines for clinical trials; 3) clinical trials; 4) submission to regulators of a dossier containing evidence on safety and efficacy; 5) creation and regulatory approval of large-scale production capacity; 6) regulatory approval of the process used to produce the vaccine; 7) vaccine production; and 8) post-marketing studies, which the regulator sometimes requires to further evaluate the vaccine and address specific questions about the vaccine’s safety, effectiveness, or possible side effects (Gomez and Robinson 2018). For non-pandemic vaccine development, these steps occur sequentially. When time is of the essence, however, steps 3), 5) and 7) can occur in parallel, albeit with significant financial risk (Thiel et al. 2021).
These development steps largely mimic those for conventional “small molecule” pharmaceuticals. But there are two important differences. The first is that the primary active ingredients in vaccines are derived, to varying degrees depending on the production method, from biological processes. The primary active ingredients in small-molecule pharmaceuticals are typically derived from chemical processes. Biologic synthesis tends to be less predictable and more subject to contamination than pharmaceuticals derived from chemical synthesis. Plotkin and colleagues note that vaccine efficacy and purity “can vary widely due to the nearly infinite combinations of biological variability in basic starting materials, the microorganism itself, the environmental condition of the microbial culture, the knowledge and experience of the manufacturing technician, and the steps involved in the purification processes” (Plotkin et al. 2017). Vaccines derived from whole viruses also need to be produced using facilities that were designed, built and equipped to separate and contain the virus (Bayot and King 2020). Second, vaccines are administered to large numbers of healthy children and adults, whereas pharmaceuticals are given to smaller numbers of individuals who experience the health condition for which the pharmaceutical is indicated. Vaccines are thus subject to more stringent safety standards, at both the testing and production phases (Mathieu 2004). Because vaccines are more complex to produce, are administered to healthy individuals, and are subject to more stringent safety and quality control standards, vaccine production tends to be more capital intensive than small-molecule pharmaceutical manufacturing (Grabowski and Vernon 1997; Scherer 2007).
Connaught 2.0 Pandemic Vaccine Development Challenges
There are several steps in the vaccine development pathway particularly relevant to the prospects of a Connaught 2.0 entity. First, there are high failure rates of R&D projects for vaccines targeting novel viral pathogens. Given the rapid development of SARS-CoV-2 (COVID) vaccines, one might infer that vaccine development for all new viruses is straightforward. However, the development of SARS-CoV-2 vaccines benefited from the confluence of several factors. These included the large body of research on MERS, SARS and other coronaviruses and the fact that the spike protein antigens for SARS-CoV-2 were relatively straightforward to develop. There are many prevalent and emerging viruses for which no vaccines exist, and not from a lack of trying. For example, attempts over the last three decades to develop a vaccine for the human immunodeficiency virus (HIV) have so far been unsuccessful. The as-yet-failed vaccine development projects for the respiratory syncytial virus, most herpes viruses, the Marburg virus and the Epstein-Barr virus provide other examples.
A recent study investigated the failure rates of vaccine candidates developed for emerging and re-emerging viral infectious diseases; all projects were initiated prior to the COVID pandemic. The study found that vaccines in phase 1 clinical trials have only a 7 percent chance of regulatory approval within 12 years (MacPherson et al. 2021). According to the WHO, a total of 235 different SARS-CoV-2 vaccine development projects were initiated after the start of the COVID pandemic, which, at the time of writing, was 24 months ago. A total of 34 candidates (only 7 percent) have achieved some form of emergency use authorization (EUA) and are being used to immunize populations in one or more countries (Shrotri et al. 2021). Only five (2.1 precent) have achieved EUA by a stringent regulator (as defined by WHO) and are being used in OECD countries. (Craven 2022; Shrotri et al. 2021).
Suppose, however, that Connaught 2.0 researchers are particularly productive, perhaps because of advances in genetic engineering and vaccine technology. In particular, suppose that 20 percent of its vaccine candidates receive regulatory approval. Under this optimistic scenario, the agency would still need to mount 13 independent vaccine R&D projects to attain a 95 percent probability of success in at least one development project.
But it would not be enough for Connaught 2.0 to develop a vaccine – it would need to do so quickly. There would be considerable public pressure to acquire the first few pandemic vaccines developed globally that meet Canadian regulatory standards. Thus, even if Connaught 2.0 were able to develop a vaccine, it would ideally do so within months of the rollout of the first approved vaccines available globally, when substantial numbers of Canadians would still need vaccinations. Only 2.1 percent of COVID vaccine development projects have resulted in a vaccine with an EUA within an OECD member country within 24 months of the start of the pandemic. This suggests that it would be risky to rely on Connaught 2.0 to be among the first organizations to develop a vaccine capable of meeting stringent regulatory standards.
Testing of Pandemic Vaccine Candidates
The second, challenging aspect of vaccine R&D is the scale and complexity of clinical trials needed to generate evidence on the safety and efficacy of new vaccines. The evidentiary standard for vaccines is typically greater than that required for conventional drugs and the US Food and Drug Administration (FDA) has among the most stringent standards globally (Jain et al. 2017; Van Norman 2016). Non-pandemic vaccines approved by the FDA over the period 2010-2020 were subject to a median of seven clinical trials, including at least two pivotal efficacy randomized trials each of which enrolled a median of 5,000 subjects (Puthumana et al. 2021).
To expedite evidence generation, COVID vaccines were given FDA emergency use authorizations based on a single large-scale efficacy randomized trial involving 30 to 45 thousand subjects (Strategic Advisory Group of Experts on Immunization (SAGE), n.d.). Presumably, future pandemic vaccine candidates would need to be tested on samples of roughly the same size.
To quickly obtain the requisite numbers, these trials have recruited subjects in multiple sites from regions with high rates of infection. For instance, the phase 3 clinical trial of the SARS-CoV-2 vaccine developed by Pfizer in partnership with BioNTech enrolled 43,661 subjects from 150 sites in the United States, Germany, Turkey, South Africa, Brazil and Argentina (Business Wire 2020).
A Connaught 2.0 entity would thus need to develop infrastructure and expertise capable of mounting a large-scale international trial shortly after it has developed its pandemic vaccine. This would be an expensive undertaking, far beyond the existing capacity of any public agency in Canada.
Thus, considerable public funds would be required to propel Connaught 2.0 to the front of the pack of organizations competing to be among the first to field an FDA or Health Canada approved pandemic vaccine. If Connaught 2.0 had no ambition to win this race, however, then development could proceed at a more relaxed pace and there would not be a need to mount a large-scale international trial. Several smaller trials – typical when testing non-pandemic vaccines – would suffice and testing costs would likely be lower. This would not solve the problem of ensuring rapid access to a pandemic vaccine but the Connaught 2.0 vaccines could still be useful if immunity to infection wanes over time, or if the virus evolves over time, creating the need for vaccines that target these variants. The relaxed vaccine testing time-frame would also accommodate the difficulty of recruiting volunteers into a placebo-controlled pandemic vaccine trial when there are approved vaccines available for use.
Pandemic Vaccine Production Capacity
The next item on Connaught 2.0’s “to do” list is to develop the capacity and expertise to produce an approved pandemic vaccine. All advocates of a proposed Connaught 2.0 agency have cited domestic vaccine production capacity as a goal. Thus, the agency would – at a minimum – be tasked with producing approved pandemic vaccines in Canada, regardless of where the vaccine originates: from in-house development or licensed from an outside organization. What would domestic production self-sufficiency entail?
To become self-sufficient, a Connaught 2.0 would require capacity to make vaccines using a variety of different vaccine technologies or “platforms.” Each of the eight major platforms is distinguished by a unique combination of equipment, biological materials and a production process. These are described in the online Appendix. Briefly, they fall under the categories: Whole Virus (live attenuated, inactivated); Protein (protein subunit, virus-like particle); Viral Vector (non-replicating viral vector, replicating viral vector); and Nucleic Acid (RNA, DNA).
The next step in the vaccine production process is to formulate a vaccine from the bulk active ingredient. Although the procedures to do so vary across the different platforms, they all involve isolating and purifying the active ingredient and formulating it so that it is suitable for administering to humans. The platforms vary in their use of adjuvants, which amplify the immune response from a given dose of the active ingredient; and any special measures needed to avoid the degradation of the active ingredient prior to its presentation to the immune system of an inoculated person. For instance, given their fragility, RNA vaccines are encapsulated in microscopic lipid particles and must be kept at extremely cold temperatures. Other vaccines use the addition of stabilizers to avoid degradation at room temperature and preservatives to prevent contamination.
The final step in vaccine production is putting the formulated vaccine into vials or syringes, labelling these so that they can be tracked, and packaging them. This step is known in the industry as “fill & finish.”
Minimum Set of Platforms Needed to Ensure Pandemic Vaccine Production Self Sufficiency
How many of the eight vaccine production platforms would Connaught 2.0 require to ensure pandemic vaccine production self-sufficiency? (See online Appendix.) This minimum set consists of those that should be sufficient to develop a vaccine for a future pandemic virus, regardless of the characteristics of the virus.
Given the success of the RNA-based COVID vaccines, one might assume that an RNA vaccine production facility is all that is needed. However, the nucleic acid and protein-based platforms were ideally suited to develop COVID vaccines. These vaccines present the immune system with a modest dose of a single protein found on the SARS‐CoV‐2 envelope. That approach may not be effective for all viruses. Vaccines for the rotavirus, for instance, require several viral proteins to evoke an immune response (with each protein coming from a different part of the virus) and the only feasible way at present to quickly formulate such a vaccine is using the whole virus platform (Ciarlet and Schödel 2009). Rotavirus vaccines also include protection against multiple strains of the virus in a single vaccine; this may be more difficult to accomplish using a nucleic acid vaccine. Moreover, not every RNA-based COVID vaccine development project was as successful as the vaccines developed by Pfizer-BioNTech and Moderna (Cohen 2021). The rapid development of the COVID-19 vaccines also benefited from research into similar coronavirus outbreaks such as SARs and MERs (Padron-Regalado 2021). Development of vaccines for a less familiar pathogen may take longer.
Our view is that to cover its bases, Connaught 2.0 would need to maintain at least three different facilities capable of producing sufficient doses to immunize the Canadian population: one facility would produce whole virus vaccines; one would produce protein-based vaccines (and/or viral vector); and one would produce nucleic acid-based vaccines.
Three distinct facilities are needed since each produces vaccines using equipment that is so specialized that it would not be feasible to repurpose a facility in short order. For instance, facilities that use fermenters to propagate viruses in cell lines, needed to produce whole virus vaccines, simply cannot be quickly repurposed to generate nucleic acid vaccines. The latter vaccines produce DNA or RNA fragments using an enzyme reaction that is more like a chemical reaction than a fermentation. Likewise, nucleic acid vaccine production facilities are not usually built in ways that ensure the containment of live viruses, making repurposing to live virus technologies challenging.
Given recent advances in vaccine platform production technology, however, it is possible to repurpose each of these three facilities so that they can switch between a limited number of different vaccines. For instance, in the past, a whole virus influenza-vaccine plant required specialized production lines tailored to the propagation of the specific influenza virus strain. That contributed to the long lead-time to produce a whole virus vaccine for another type of virus. Newer whole virus technologies, however, use a common virus propagation platform (such as vero cells) that can produce a variety of different types of vaccines.
This reduces both cost and time needed to produce new whole virus vaccines. The advancements in platform technologies enable the use of more flexible and modular plants; we elaborate on this later in the paper.
It is also possible that given the sustained research focus on protein- and nucleic acid based vaccine production platforms, these will eventually be capable of producing vaccines that are currently dependent on whole virus platforms (Pardi et al. 2018; Salzer et al. 2021). Until then, it would be prudent to maintain at least three different platforms.
The cost of building these three facilities and obtaining regulatory certification is not known with precision. We can rely, however, on the publicly disclosed production costs of facilities that have been recently built, or are currently being built, to get ballpark estimates. These commercial-scale facility costs are in the order of $500 million to $1 billion. Specifically, Plotkin et al. (2017) estimate that the cost of a whole virus vaccine plant is between US$50 to $500 million per antigen depending on the complexity of design, automation, segregation, utilities, and contamination controls, and as much as US$700 million for multiple vaccines. Sanofi’s new egg-based whole virus plant at its Connaught campus is expected to cost C$925 million to construct and certify (Sanofi Canada 2021). Lonza’s vaccine and biologics contract facility in Switzerland cost US$715 million (Kansteiner 2021). Novartis’ cell-based influenza vaccine plant, subsequently purchased by Sequiris, cost US$1 billion (Pharmaceutical Technology n.d.).
These costs, while considerable, would certainly be manageable for a high-income country like Canada. Operating these facilities would be the larger hurdle. Connaught 2.0 would require personnel capable of producing vaccines in each facility using procedures that meet the most current Good Manufacturing Practices (cGMP) and sterility requirements (Barone et al. 2020). To attain the requisite expertise producing in each platform, practically this means that the plants would need to produce vaccines continuously, at or near full capacity. Vaccine production is a skill that is mastered through experience; production teams cannot sit idle for extended periods only to initiate full-scale production on demand (Dunleavy 2021b; Moutinho and Wadman 2021; Plotkin et al. 2017).
The production facilities would also need a secure supply chain for chemicals, culture media, tubing, chromatography media, syringes, and other key inputs. These need to be sourced domestically or otherwise assured. The lack of a backup for one piece of critical material can shut down an operation for weeks or months. For instance, Novovax’s COVID vaccine production has had costly delays because a key input – sterile manufactured bags were not available (Dunleavy 2021a). Supply contracts are presumably more secure, the longer the contract duration. A Connaught 2.0 facility that was dormant for many months, and suddenly called into action may have difficulty sourcing inputs.
In short, not knowing in advance which vaccine production platform will be required, Connaught 2.0 would need to create capacity to manufacture using several different vaccine platforms. Each of the platforms and production processes used in them would require regulatory certification. The plants would need to be run at near full capacity during non-pandemic times to hone production processes, meet evolving regulatory standards and maintain input supply contracts. To do so, presumably, it would produce some or all the 21 different vaccines used in Canadian immunization programs, such as pediatric, human papillomavirus and seasonal influenza vaccines. It might also produce COVID-type vaccines should variants of SARS‐CoV‐2 periodically circulate in the population.
The use of Connaught 2.0 to ensure Canada’s pandemic vaccine supply security has several drawbacks. First, Connaught 2.0 would necessarily be a large public agency; in addition to its physical plant, it would need to operate regulatory affairs, legal, procurement, finance, human resources, R&D and the various other departments housed in commercial vaccine companies. The literature suggests that there are aspects of large state or publicly owned enterprises that make them more costly to operate than privately owned businesses, even after controlling for market structure (Boardman and Vining 1989; Goldeng et al. 2008; Shirley and Walsh 2001). In particular, the corporate governance literature suggests that managers of state-owned enterprises have weaker incentives to perform, and that their principals have less efficient means of monitoring the managers, especially if they can rely on public funds to cover shortfalls (Goldeng et al. 2008; Tasker 2021d).
Second, Connaught 2.0 would presumably compete alongside private companies for market share. For example, it might submit bids in the competitive tenders organized by Public Services and Procurement Canada to provide seasonal influenza and pediatric vaccines (Cutcliffe 2010; Keelan 2000). If Connaught 2.0 were awarded the contracts, this would displace sales by the commercial vaccine companies that operate in Canada: Sanofi, GSK and the smaller firms that are now entering the industry (Government of Canada 2021). This would certainly not enhance Canada’s private sector vaccine production capacity (Cutcliffe, 2010), and would considerably raise the stakes for Connaught 2.0.
Third, the vaccine market in Canada is much smaller than the minimum efficient scale of bulk vaccine manufacturing facilities, especially facilities that use the whole virus platform. GSK’s seasonal influenza vaccine plant (located in Laval), for instance, has an annual production capacity several times the size of Canada’s annual demand. Thus, if it produces solely for the Canadian market, Connaught 2.0’s average per vial production costs would be markedly higher than the average costs incurred by commercial producers that currently serve Canadian and foreign markets.
Finally, having a publicly owned producer could add political considerations in public health decisions, which can undermine public confidence in vaccines. For example, if the government invests heavily in the development, approval and production of a pandemic vaccine, there would be pressure to adopt that vaccine, even if rival vaccines might have better safety and efficacy.
Australia’s Experience with COVID Vaccine Procurement
Australia’s experience illustrates the challenges of developing pandemic vaccines. Whereas Canada entered into supply contracts with a variety of different international vaccine producers, Australia initially focussed on developing domestic capacity to manufacture a COVID vaccine. In September 2020, the Australian government contributed AUS$1.4 billion towards the construction of manufacturing facilities for two COVID vaccines that were then under development: a vaccine co-developed by CSL, an Australian biotechnology company and the University of Queensland; and a vaccine co-developed by AstraZeneca and Oxford University (Morrison et al. 2020).
CSL had its origins as a public agency called the Commonwealth Serum Laboratory that produced vaccines, anti-venoms, and blood products. The Australian government divested this agency in 1994 (Quiggin 2020); under private ownership, CSL has grown into a leading global biotech company with annual revenues in the order of US$ 9 billion. (CSL n.d.).
The CSL vaccine candidate was abandoned in phase 1 trials. The AstraZeneca vaccine was approved by the Australian regulator but its production was slowed down by manufacturing problems (Evershed and Nicholas 2021). More recently, evidence emerged of a rare but life-threatening side effect from the AstraZeneca vaccine: blood clots, which obstruct blood vessels (thrombosis) combined with low blood platelet count (thrombocytopenia) which can cause internal bleeding (Cattaneo 2021). This side effect risk is most pronounced in those under 50 years of age. Unable to repurpose the existing vaccine manufacturing capacity to produce mRNA vaccines (Koehn 2021), the Australian government entered into supply agreements with Pfizer to provide vaccines for the under-50 population (Australian Government, Department of Health, n.d.). These supply issues slowed down the campaign to vaccinate the Australian population. As of May 24, 2021, Australia had administered only 3.56 million, vaccine doses; Canada with one-and-a-half times the population had administered 20.7 million, or nearly six times as much.
CSL has subsequently committed to manufacture the AstraZeneca vaccine (CSL 2021), but it is no longer the vaccine of choice. Australia and New Zealand have recently licensed Novavax COVID-19 vaccine in partnership with Biocelect, a local Australian company. Australia has also entered into an agreement in principal with Moderna to build a large-scale mRNA vaccine facility by 2024 (Arthur 2021b).
Leveraging and Enhancing Canada’s Existing Capacity
Our view is that Canada can achieve greater pandemic vaccine security at lower cost by contracting with academic and commercial players in the vaccine sector. As evidence in support of our position, we note that the COVID vaccines currently used in the G7 member countries (Canada, France, Germany, Italy, Japan, the United Kingdom and the United States) are provided by for-profit firms – AstraZeneca, in partnership with Oxford University; a partnership between Pfizer and BioNTech; Moderna; and Johnson & Johnson. Thus, private, for-profit organizations – not public enterprises – have emerged as the leading pandemic vaccine suppliers in the most advanced economies. This speaks to their considerable capacity to develop (including via partnerships with biotech firms and academic labs that have promising technologies), test and produce vaccines in compressed timelines. A company with this capacity is necessarily large; Sanofi’s vaccine division, for instance, has 15,000 employees globally. It has about 1,000,000 litres of fermenter capacity in 27 facilities worldwide. We also note that our proposal is not entirely novel. Canada previously contracted with GSK to provide reserve capacity for the production of pandemic influenza vaccines (Henry et al. 2017).
What capacity does Canada need to enhance? The primary bottleneck in the pandemic vaccine development pathway is not the discovery and testing of new vaccines. Globally there are 235 different COVID vaccine development projects at different stages (McDonnell et al. 2020), including the five approved for use (albeit on an emergency basis) in Canada (Government of Canada n.d.). If Canada can license vaccines developed for future pandemics (regardless of where the vaccines are developed), then attention can focus on the actual bottleneck – domestic production capacity. We currently rely on foreign producers for most of our pharmaceutical and vaccine requirements. Indeed, Industry Canada reports that “in 1973, approximately 19 percent of Canada’s domestic demand for vaccines and therapeutic drugs was supplied through imports. Today, Canada imports 85 percent of its requirements, primarily from Germany, Switzerland, and the United States” (Government of Canada 2021).
Thus, expansion of domestic vaccine production capacity is required. As was noted earlier, vaccine production can be divided into two stages, 1) production of bulk quantities of the vaccine and 2) bottling and labelling, known in the industry as “fill & finish.” If foreign production bottlenecks occur at the fill & finish stage, then it would be sufficient for Canada to acquire capacity and experienced personnel needed to fill & finish in Canada using bulk vaccines acquired internationally.
There are two large-scale vaccine filling facilities in Canada. The first is the GSK facility in Ste. Foy and the other is Sanofi’s Connaught facility; this latter site is being upgraded. There are also some smaller contract manufacturing organizations (CMOs) that have received federal government funds to help expand capacity (Innovation, Science and Economic Development Canada 2021b; Novocol Pharma 2021). Despite these capacity increases, however, Canada will be unable to fill millions of doses of a pandemic vaccine in a compressed time-frame. If Canada wishes to become self-sufficient in fill & finish, additional capacity will be required.
If foreign vaccine production bottlenecks also occur at the first stage, then Canada would also need capacity and experienced personnel to produce vaccine in some or all the three platforms described earlier. The actual number of platforms for which Canada needs expanded capacity depends on whether Canada elects to enter into trade agreements with other countries to share production. The terms of an agreement would depend on the number and capacities of the partner countries, but one could imagine an agreement between, say, the UK, Australia and Canada could require each to specialize in one production platform and create sufficient capacity to supply vaccines for the population of all three countries. (It would also be desirable to have additional capacity to meet some of the vaccine needs of lower-income countries.) Agreements that also involve smaller countries, like New Zealand, could allow such countries to meet their obligations through cash transfers to producing countries. The countries that Canada could partner with would most likely be the advanced industrialized economies (such as the members of the G20) that would benefit from trade. This may rule out the EU member countries and the US, both of which appear to be already self-sufficient in the three vaccine production platforms.
Canada has several options to expand platform-specific production capacity. The first is to acquire reserve capacity in domestic production facilities that routinely make large volumes of the vaccine using the platform. The contract would require the producer to switch production to the pandemic vaccine should the need arise. Thus, a facility that produces, say, mRNA vaccines for one pathogen would need to switch to an mRNA or DNA-based vaccine for the pandemic pathogen should this platform be required. This changeover and the requisite regulatory certification would require several months; but the facility personnel will be familiar with the production technology and the producer will have existing input supply contracts. This approach does require that the facility cease production of the vaccines that normally are made. To avoid supply disruptions, the facility would need to routinely maintain stockpiles of these vaccines; the capacity of the facility might also need to be upgraded. The cost of doing so would need to be built into the contract price.
The second way that Canada can obtain pandemic vaccine production capacity is to construct modular manufacturing facilities that could be quickly adapted to produce vaccines for a novel pathogen, while also meeting all the strict requirements for this type of facility (Labant 2021). This would most practically involve clean room space for a new production line within an existing cGMP-certified manufacturing facility. The “plug and play” technology already exists. The Swiss vaccine maker Lonza has used its modular facilities in Basel to quickly ramp up production of Moderna’s mRNA COVID vaccine.
These modular facilities could be equipped with the requisite bioreactors, purification and formulation machinery for vaccine production using one of the preferred pandemic platforms. The facility would be located on the grounds of an established vaccine producer and would tap into the producer’s sterile water, gas, steam, ventilation, waste disposal and other utilities/infrastructure. This producer would be responsible for operating and maintaining the facility to keep it in a state of readiness and full cGMP compliance while providing a roster of experienced personnel capable of operating it at full capacity when needed for production of a pandemic vaccine.
This modular approach is less useful for whole virus vaccines – the fermenters and containment systems needed to propagate viruses do not lend themselves to a “plug and play” approach. Thus, domestic pandemic vaccine production self-sufficiency would require that there be reserve capacity in an existing functional whole=virus production facility.
To summarize, Canada has two options to secure domestic commercial-scale pandemic vaccine manufacturing capacity. It can do so either by contracting for reserve capacity or by building modular surge capacity on an existing vaccine manufacturing site, or by using a combination of both. The commercial partner could be one of the large multinational vaccine producers that either have existing capacity in Canada or are planning to establish capacity in Canada. The commercial partner could also be a domestic CMO that routinely produces for multinational vaccine companies.
Each of these contracting approaches would require substantial public investments. Nevertheless, our sense is that these approaches would be ultimately less expensive than a Connaught 2.0 approach given that the Connaught 2.0 facilities would need to operate continuously to maintain production readiness and its overhead costs would in large part duplicate costs already incurred by the commercial suppliers in Canada.
What production capacity does Canada currently have in each of these different vaccine platforms? Table 1 reports on the existing and planned capacity in each of the three platforms. This existing and planned manufacturing capacity is housed in four different types of organizations.
The first of these are the multinational pharmaceutical companies with vaccine manufacturing sites in Canada: Sanofi in Toronto and GSK in Ste. Foy. These multinationals have the most experience with large-scale vaccine manufacturing capacity in Canada. A third multinational, Medicago, is constructing a production facility in Quebec City that is expected to enter service in 2023 (Medicago 2020). All three sites are or will be capable of producing influenza vaccines in response to an influenza pandemic in quantities well beyond the needs of Canada. The Sanofi facility also produces combination vaccines for export to OECD countries and the Medicago facility will be capable of producing plant-based virus-like-particle (VLP) protein subunit vaccines.
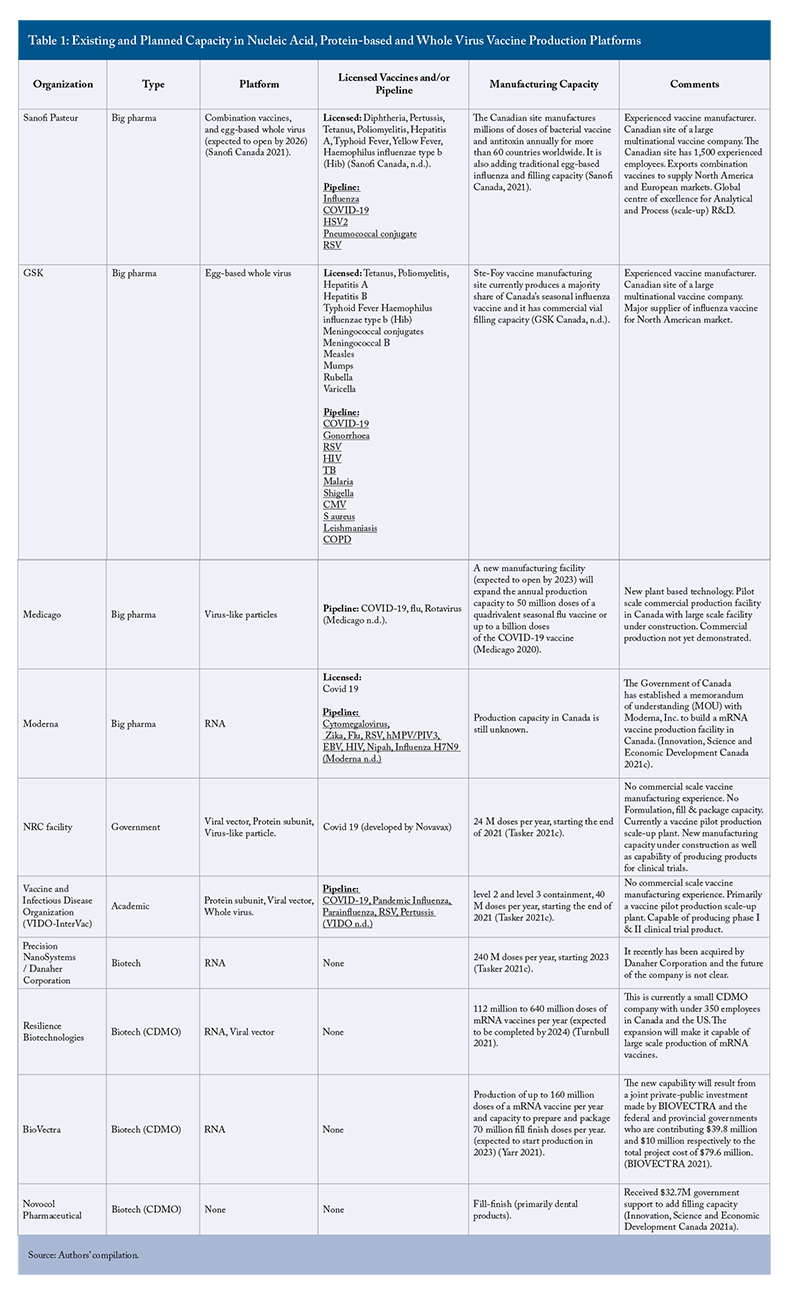
Both GSK and Sanofi have large-scale vaccine formulation and fill & finish operations beyond the capacity needed for Canada and Sanofi also has a vaccine scale-up/pilot facility capable of producing clinical trial material for large multinational clinical trials. Canada could contract with GSK and Sanofi to fill & finish vaccines for the next pandemic. It is unclear, however, if these fill & finish facilities have the capacity to quickly produce the large number of vials of any future pandemic vaccine. (Moreover, even if they had the capacity, they might be not available to fill & finish pandemic vaccines.) Thus, additional capacity will likely be needed.
The second type of organization in Canada’s vaccine sector are the small to medium-sized biotechnology companies and contract development and manufacturing organizations (CDMOs). Biotech and CDMO firms in Canada have no licensed vaccines and hence little vaccine manufacturing experience at a commercial scale but some are in the process of building large-scale RNA-based production platforms that will be completed within the next two to three years. For example, Resilience, a Californian-headquartered CDMO, is creating capacity to produce mRNA vaccines for Moderna in Mississauga, Ontario (Arthur 2021a).
The third organization is the Vaccine and Infectious Disease Organization (VIDO), based at the University of Saskatchewan. VIDO has an intermediate-scale, pilot vaccine production facility capable of producing clinical trial material. This organization has extensive vaccine development expertise and is hoping to expand to full-scale manufacturing with a new facility funded by the Canadian government. When this facility is completed, this organization would likely become a CDMO, manufacturing vaccines on behalf of other companies.
The fourth organization in Canada’s vaccine ecosystem is the National Research Council (NRC), an agency of the government of Canada. The NRC is currently completing a vaccine production facility in Montreal. When operational, it will be a small to medium-size bulk-vaccine-production facility capable of producing vaccines in at least two platforms concurrently. Its capacity of 24 million doses/year is too small to produce pandemic vaccines for Canada, let alone a global market, especially if two or three doses are required. It could, however, produce pandemic vaccines for healthcare workers, the immunocompromised and other priority groups. NRC’s vaccine production arm will likely become another CDMO.
Moving Forward
Should Canada adopt our recommendations, it would first need a public agency and pandemic vaccine strategy. The agency’s mandate would include: i) a survey of existing and planned domestic vaccine production and filling capacity; ii) discussions with other countries that might be interested in entering into a mutual pandemic vaccine supply agreement; iii) depending on the outcome of these discussions, an assessment of what additional domestic capacity (if any) is needed; and finally, iv) negotiations with commercial vaccine producers (major multinationals and CDMOs) that are capable of building this capacity and employing it during non-pandemic times.
To do so, government personnel would need to work with technical experts, including academics and biotech and pharmaceutical industry stakeholders who have experience in commercial-scale vaccine production. It would also need experts to help negotiate the contracts with the commercial vaccine producers and academic consortia (such as the vaccine alliance between the University of Saskatchewan’s VIDO and Canada’s Global Nexus for Pandemics and Biological Threats, based at McMaster University (Giles 2021)). The agreements would need to cover:
- which party, the Government of Canada or the producer, would handle the in-licensing of the vaccine technology, if needed?
- the amount of reserve capacity required and the additional construction, if any, needed to obtain this capacity;
- the size of the stockpiles of the vaccine normally produced by the contracting supplier of reserve capacity;
- the specifications of the modular facility, should this option be pursued, and expectations regarding the maintenance and updating of the production capabilities as the vaccine production technology changes; and
- the amount paid to suppliers to carry out the contracts.
The Government of Canada does not have a department or agency that currently engages in this work. The United States does; it is called the Biomedical Advanced Research and Development Authority (BARDA). BARDA works with academia and industry to develop vaccines, drugs, therapies, and diagnostic tools for a range of public health medical emergencies: chemical, biological, radiological, and nuclear accidents, incidents and attacks; as well as pandemic influenza, and emerging infectious diseases. BARDA entered into 81 contracts to aid development of COVID vaccines, therapeutics and diagnostics. The BARDA model could be used to help structure the new Canadian agency.
One question is where the new pandemic vaccine production group should be housed in the federal civil service. One possible home for the pandemic vaccine production group is the National Research Council (NRC). The NRC’s mandate is to undertake, assist and promote scientific and industrial research in fields of importance to Canada. Indeed, the NRC’s Industrial Research Assistance Program (NRC IRAP) is partnering with Immunovaccine Technologies, Entos Pharmaceuticals, Providence Therapeutics, Glycovax Pharma, Symvivo, and Biodextris in the development of COVID vaccines (see “Funding domestic vaccine and therapeutic candidates as part of the Government of Canada’s COVID-19 response” 2020). The NRC is more recently involved in building capacity to manufacture pandemic vaccines for Novavax and with modular capacity for other clinical development scale work (National Research Council Canada 2021).
One limitation with using the NRC is that its focus is industrial research and not emergency preparedness. A more natural home for pandemic preparedness planning and strategy is the Centre for Emergency Preparedness and Response (CEPR), which is a division of the Public Health Agency of Canada. CEPR monitors disease outbreaks globally, develops public health protocols and manages the National Emergency Strategic Supply –- a stockpile of vaccines, personal protective gear and related material needed during pandemics.
Conclusion
The frequency of viral outbreaks and pandemics in the last two decades, including the current COVID pandemic, has focussed attention on Canada’s pandemic preparedness generally and its pandemic vaccine development and production self-sufficiency, in particular. As Canada faced disruptions in the supply of COVID vaccines from other countries, there were numerous calls for a public agency that would be charged with pandemic vaccine production and possibly vaccine development as well.
We reviewed the prospects for such an agency and noted that it would be risky to rely on it to rapidly develop and test a vaccine given the high failure rates for vaccine development projects and logistical challenges in mounting a large-scale international multi-centre trial in short order. But a public agency that instead focussed on producing vaccines licensed from domestic or international developers would also face challenges. The primary issue is one of production readiness. Practically, production facilities need to be operating continuously at or near full-scale capacity to hone the processes needed to meet stringent and evolving regulatory standards and ensure personnel have sufficient experience. Facilities also need a reliable supply of key inputs. The public agency would thus need to be engaged in full-scale vaccine production even in non-pandemic times to maintain both production know-how and stable input supply chains. It would need to do so in each of the three production platforms that may be needed to produce vaccines for the next pandemic virus. This raises the question of what vaccines the agency would routinely produce, in non-pandemic times, and where the vaccines would be distributed. If it elects to compete with existing private-sector vaccine producers for domestic or international sales, that will certainly not increase private investment in vaccine manufacturing capacity and may degrade it.
Our view is that Canada can achieve a more reliable supply of vaccines for future pandemics, and at lower cost, by contracting with existing commercial producers that are already engaged in continuous and full-scale production and who thus have demonstrated technical competency and have secure input supply chains. We thus concur with the recommendation of David Naylor and colleagues, in their 2003 report, Learning from SARS that
The Government of Canada should foster workable public-private partnerships with the biotechnology, information technology, and pharmaceutical industries for shared research interests in the realm of emerging infectious diseases, including new vaccines …. (National Advisory Committee on SARS and Public Health 2003.)
Canada can work with the large multinationals, including Sanofi and GSK, and the smaller domestic CDMOs that produce on behalf of a multinational. The government can purchase either reserve capacity in existing domestic production facilities or can cover the cost of an adjacent modular production facility that can thus tap into the ventilation, steam, gas and other utilities needed to run the facility. This latter “plug and play’’ approach at present only lends itself well to the newer nucleic acid and protein-based vaccine platforms. Canada would still need to contract for reserve capacity with an established vaccine maker, such as GSK, Sanofi, or even Moderna, if it succeeds with its vaccine development projects. The number and capacity of the vaccine manufacturing platforms that Canada requires depends on whether Canada can negotiate an agreement with other countries that allows each country to specialize in a platform and share pandemic vaccines with partners should the need arise. Regardless of its approach, however, Canada needs to act soon if we are to be ready for the next pandemic.
References
Abbott, A. 2021. “COVID’s mental-health toll: how scientists are tracking a surge in depression.” Nature, 590(7845): 194–195. pubmed.ncbi.nlm.nih.gov.
Adam, D. 2022. “The pandemic’s true death toll: millions more than official counts.” Nature, 601(7893): 312–315.
Arthur, R. 2021a. Resilience to manufacture mRNA for Moderna in Canada. BioPharma. September 8. https://www.biopharma-reporter.com/Article/2021/09/08/Resilience-to-manufacture-mRNA-for-Moderna-in-Canada#:~:text=National%20Resilience%2C%20Inc.%20will%20manufacture,its%20facility%20in%20Mississauga%2C%20Ontario.
________. 2021b. Moderna to build mRNA vaccine manufacturing facility in Australia. BioPharma. December 14. https://www.biopharma-reporter.com/Article/2021/12/14/Moderna-to-build-mRNA-vaccine-manufacturing-facility-in-Australia
Australian Government, Department of Health. (n.d.). Australia’s vaccine agreements. Retrieved May 26, 2021, from https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/covid-19-vaccine-government-response/australias-vaccine-agreements
Barone, P. W., Wiebe, M. E., Leung, J. C., Hussein, I. T. M., Keumurian, F. J., Bouressa, J., Brussel, A., Chen, D., Chong, M., Dehghani, H., Gerentes, L., Gilbert, J., Gold, D., Kiss, R., Kreil, T. R., Labatut, R., Li, Y., Müllberg, J., Mallet, L., … Springs, S. L. 2020. “Viral contamination in biologic manufacture and implications for emerging therapies.” Nature Biotechnology, 38(5): 563–572.
Barreto, L., and Rutty, C. J. 2002. “The speckled monster. Canada, smallpox and its eradication.” Canadian Journal of Public Health. Revue Canadienne de Sante Publique, 93(4): I1-20.
Barris, T. 2021. Cure within our grasp. Tedbarris.Com. February 4. https://tedbarris.com/2021/02/04/cure-within-our-grasp/
Barry, K. 2021. “Make the vaccines public.” Canadian Dimension. January 26.
Bayot, M. L., and King, K. C. 2020. “Biohazard Levels.” In StatPearls. StatPearls Publishing.
Bhatt, K., Agolli, A., Patel, M. H., Garimella, R., Devi, M., Garcia, E., Amin, H., Domingue, C., Del Castillo, R. G., and Sanchez-Gonzalez, M. 2021. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries, 9(1). https://www.ncbi.nlm.nih.gov/pmc/articles/pmc8137279/
BIOVECTRA. 2021. “BIOVECTRA mRNA Vaccine and Bio-manufacturing Facility Announced.” https://www.biovectra.com/biovectra-mrna-vaccine-and-bio-manufacturing-facility-announced/
Boardman, A. E., and Vining, A. R. 1989. “Ownership and Performance in Competitive Environments: A Comparison of the Performance of Private, Mixed, and State-Owned Enterprises.” The Journal of Law and Economics, 32(1): 1–33.
Business Wire. 2020. “Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints.” November 18. Business Wire. https://www.businesswire.com/news/home/20201118005595/en/
Campbell, B. S. 2020. Feds need to double down to ensure universal access to coronavirus vaccines. National Observer. July 22.
Carroll, D., Daszak, P., Wolfe, N. D., Gao, G. F., Morel, C. M., Morzaria, S., Pablos-Méndez, A., Tomori, O., and Mazet, J. A. K. 2018. “The Global Virome Project.” Science, 359(6378): 872–874.
Cattaneo, M. 2021. “Thrombosis with Thrombocytopenia Syndrome associated with viral vector COVID-19 vaccines.” European Journal of Internal Medicine. https://doi.org/10.1016/j.ejim.2021.05.031
Ciarlet, M., and Schödel, F. 2009. “Development of a rotavirus vaccine: Clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq®.” Vaccine, 27, G72–G81.
Cohen, J. 2021. What went wrong with CureVac’s highly anticipated new mRNA vaccine for COVID-19? https://www.sciencemag.org/news/2021/06/what-went-wrong-curevac-s-highly-anticipated-new-mrna-vaccine-covid-19
Council on Foreign Relations. (n.d.). Major Epidemics of the Modern Era. Cfr.Org. Retrieved September 24, 2021, from https://www.cfr.org/timeline/major-epidemics-modern-era
Craven, J. 2022. COVID-19 vaccine tracker. Regulatory Affairs Professionals Society (RAPS). February 11. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
CSL. (n.d.). Our Company. CSL. https://www.csl.com/our-company
CSL. 2021. “CSL Reaffirms Commitment to Manufacture AstraZeneca COVID Vaccine into 2022.” CSL. October 14. https://www.csl.com/news/2021/20211014-csl-reaffirms-commitment-to-manufacture-astrazeneca-covid-vaccine-into-2022
Cutcliffe, N. 2010. “Pathway to Access: Manufacturing, Supply, and Procurement Systems.” BIOTECanada.
Cutler, D. M., and Summers, L. H. 2020. “The COVID-19 Pandemic and the $16 Trillion Virus.” JAMA: The Journal of the American Medical Association, 324(15): 1495–1496.
Danzon, P. M., and Sousa Pereira, N. 2011. “Vaccine Supply: Effects of Regulation and Competition.” International Journal of the Economics of Business, 18(2): 239–271.
Darrah, D. 2020. “By Cutting Big Pharma Out of Vaccine Production, We Can Help Neutralize Anti-Vaxx Paranoia.” Jacobin. December 23.
Dawood, F. S., Iuliano, A. D., Reed, C., Meltzer, M. I., Shay, D. K., Cheng, P.-Y., Bandaranayake, D., Breiman, R. F., Brooks, W. A., Buchy, P., Feikin, D. R., Fowler, K. B., Gordon, A., Hien, N. T., Horby, P., Huang, Q. S., Katz, M. A., Krishnan, A., Lal, R., …Widdowson, M.-A. 2012. “Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study.” The Lancet Infectious Diseases, 12(9): 687–695.
Defries, R. D. 1968. The First Forty Years, 1914-1955: Connaught Medical Research Laboratories, University of Toronto. University of Toronto Press.
Deveaux, G. 2021. “The ‘free market’ has failed Canada on pharmaceutical development.” The Hamilton Spectator. April9.
Dunleavy, K. 2021a. “Plastic bag shortage a hefty problem for Novavax’s COVID-19 vaccine production push.” Fierce Pharma. April 13.
________. 2021b. “Emergent shuts down COVID-19 vaccine production at troubled plant after feds put J&J in charge.” Fierce Pharma. April 19.
Editorial, The Times Colonist. 2021. Editorial: “Vaccine production belongs in public hands.” The Times Colonist. February 27.
Evershed, N., and Nicholas, J. 2021. “Australia has received only 70% of Covid vaccine doses the government expected by now.” Governmn The Guardian. April 23.
Government of Canada. https://nrc.canada.ca/en/stories/funding-domestic-vaccine-therapeutic-candidates-part-government-canadas-covid-19-response
Giles, D. 2021. “VIDO, Global Nexus teaming up to fight COVID-19, future pandemics.” Global News. February 25.
Goldeng, E., Grünfeld, L. A., and Benito, G. R. G. 2008. “The Performance Differential between Private and State Owned Enterprises: The Roles of Ownership, Management and Market Structure.” Journal of Management Studies, 45(7): 1244–1273.
Gomez, P. L., and Robinson, J. M. 2018. “Vaccine Manufacturing.” In S. A. Plotkin, W. A. Orenstein, P. A. Offit, and K. M. Edwards (Eds.), Plotkin’s Vaccines (Seventh Edition pp. 51-60.e1). Elsevier.
Gopinath, G. 2020. “The Great Lockdown: Worst Economic Downturn Since the Great Depression.” IMFBlog. https://blogs.imf.org/2020/04/14/the-great-lockdown-worst-economic-downturn-since-the-great-depression/
Government of Canada. (n.d.). Vaccines for COVID-19: Authorized vaccines. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines.html
________. 2021. Consultation document: Considering the creation of new biomanufacturing capacity for Canada. Government of Canada. March 16. https://www.ic.gc.ca/eic/site/151.nsf/eng/00002.html#9
________. 2020. Funding domestic vaccine and therapeutic candidates as part of the Government of Canada’s COVID-19 response. October 1.
Grabowski, H. G., and Vernon, J. M. 1997. The Search for New Vaccines: The Effects of the Vaccines for Children Program. AEI Press.
GSK Canada. (n.d.). GSK in Canada. https://ca.gsk.com/en-ca/about-us/gsk-in-canada/
Henry, B., Gadient, S., and Canadian Pandemic Influenza Preparedness (CPIP) Task Group. (2017). Canada’s pandemic vaccine strategy. Canada Communicable Disease Report = Releve Des Maladies Transmissibles Au Canada, 43(7–8), 164–167.
Herder, M., and Murthy, S. 2021. “A ‘once-in-a-generation’ chance to reset vaccine innovation in Canada.” The Hill Times. April 19.
Holland, M. S. 2017. “Liability for vaccine injury: the United States, the European Union, and the developing world.” Emory LJ, 67, 415.
Innovation, Science and Economic Development Canada. 2021a. “Major investments in domestic firms to rebuild Canada’s biomanufacturing sector.” https://www.canada.ca/en/innovation-science-economic-development/news/2021/03/major-investments-in-domestic-firms-to-rebuild-canadas-biomanufacturing-sector.html
________. 2021b. “Government of Canada continues to strengthen Canada’s biomanufacturing sector.” https://www.canada.ca/en/innovation-science-economic-development/news/2021/05/government-of-canada-continues-to-strengthen-canadas-biomanufacturing-sector.html
________. 2021c. “Government of Canada announces agreement with leading COVID-19 vaccine developer Moderna, Inc. to build mRNA vaccine facility in Canada.” https://www.canada.ca/en/innovation-science-economic-development/news/2021/08/government-of-canada-announces-agreement-with-leading-covid-19-vaccine-developer-moderna-inc-to-build-mrna-vaccine-facility-in-canada.html
Institute of Medicine (US). 2003. Financing Vaccines in the 21st century: Assuring Access and Availability. National Academies Press.
Jackson, N. A. C., Kester, K. E., Casimiro, D., Gurunathan, S., and DeRosa, F. 2020. “The promise of mRNA vaccines: a biotech and industrial perspective.” NPJ Vaccines, 5(1): 11.
Jain, A. B., Mollet, A., and Szucs, T. D. 2017. “Regulatory watch: Structural and procedural characteristics of international regulatory authorities.” Nature Reviews. Drug Discovery, 16(9): 594.
Kansteiner, F. 2021. “Lonza plots $935M in capacity upgrades in Europe and U.S., plans to add 550 jobs over next 3 years.” Fierce Pharma. May 6.
Keelan, J. E. 2000. Concurrency in Public Health Governance: The Case of the National Immunization Strategy. Institute of Intergovernmental Relations, Queen’s University.
Koehn, E. 2021.. “‘Not infinite’: CSL unable to quickly flick switch on vaccine plans.” Sydney Morning Herald. April 9.
Labant, M. 2021. “The Sleeping Giants of Vaccine Production Awaken.” Genetic Engineering & Biotechnology News, 41(2): 34–36, 38.
Last, J. M., Bator, P. A., and Rhodes, A. J. 1991. “Within reach of everyone: A history of the University of Toronto school of hygiene and the Connaught laboratories, volume 1, 1927 to 1955.” Journal of Public Health Policy, 12(3): 401.
Lexchin, J. 2020. “‘Vaccine nationalism’ could threaten Canada’s access to a COVID-19 vaccine.” Global News. July 12.
Loftus, P., and Vieira, P. “Vaccine Manufacturing Issues Force Moderna to Cut Supplies to Canada, U.K.” The Wall Street Journal. April 16.
MacIntyre, C. R., and Bui, C. M. 2017. “Pandemics, public health emergencies and antimicrobial resistance - putting the threat in an epidemiologic and risk analysis context.” Archives of Public Health = Archives Belges de Sante Publique, 75: 54.
MacPherson, A., Hutchinson, N., Schneider, O., Oliviero, E., Feldhake, E., Ouimet, C., Sheng, J., Awan, F., Wang, C., Papenburg, J., Basta, N. E., and Kimmelman, J. 2021. “Probability of Success and Timelines for the Development of Vaccines for Emerging and Reemerged Viral Infectious Diseases.” Annals of Internal Medicine, 174(3): 326–334.
Mathieu, M. P. 2004. Biologics Development: A Regulatory Overview. PAREXEL International Corporation.
Mayer, C. 2020. “Privatized Connaught Labs is still going strong.” Financial Post. December 10. https://financialpost.com/opinion/charlie-mayer-privatized-connaught-labs-is-still-going-strong
McDonnell, A., Van Exan, R., Lloyd, S., Subramanian, L., Chalkidou, K., La Porta, A., Li, J., Maiza, E., and Reader, D. 2020. “COVID-19 Vaccine predictions: using mathematical modelling and expert opinions to estimate timelines and probabilities of success of COVID-19 vaccines.” Washington (DC): Center for Global Development. Https://Www. Cgdev. Org/Sites/Default/Files/COVID-19-Vaccine-Predictions-Full. Pdf (Last Accessed: 24. 10. 2020). https://covid19.ariadnelabs.org/wp-content/uploads/sites/6/2020/10/COVID-19-Vaccine-Predictions-Full.pdf
McQuaig, L. 2020a. “The public lab that could have helped fight COVID-19 pandemic.” Toronto Star. March 11.
McQuaig, L. 2020b. “Steps Must Be Taken to Nationalize Pharmaceutical Production in Canada.” Broadbent Institute. June 25.
Medicago. (n.d.). Pipeline. https://www.medicago.com/en/pipeline/
Medicago. 2020. “Medicago announces positive results in animal trials for its vaccine candidate against COVID-19.” https://www.medicago.com/en/media-room/medicago-announces-positive-results-in-animal-trials-for-its-vaccine-candidate-against-covid-19/
Moderna. (n.d.). Moderna’s Pipeline. https://www.modernatx.com/pipeline
Morrison, S., Hunt, G., and Andrews, K. 2020. “Australia secures onshore manufacturing agreements for two COVID-19 vaccines.” https://www.pm.gov.au/media/australia-secures-onshore-manufacturing-agreements-two-covid-19-vaccines
Moutinho, S., and Wadman, M. 2021. “Brazil and Russia face off over vaccine contamination charge.” Science, 372(6542): 554.
Nabel, G. J. 2008. “The development of gene-based vectors for immunization.” Vaccines, 1393.
National Advisory Committee on SARS and Public Health. 2003. Learning from SARS: Renewal of Public Health in Canada. National Advisory Committee on SARS and Public Health.
National Research Council Canada. 2021. COVID-19 response: Building the infrastructure. Government of Canada. February 2. https://nrc.canada.ca/en/covid-19-response-building-infrastructure
NDP. 2020. “NDP calls on the government to get back in the business of making vaccines.” NDP. December 2. https://www.ndp.ca/news/ndp-calls-government-get-back-business-making-vaccines
Novocol Pharma. 2021. “Novocol Pharma Receives Contribution from the Government of Canada.” https://novocolpharma.com/novocol-pharma-receives-contribution-from-the-government-of-canada/
Padron-Regalado, E. 2021. “Correction to: Vaccines for SARS-CoV-2: Lessons from Other Coronavirus Strains.” Infectious Diseases and Therapy, 10(1): 631.
Paget, J., Spreeuwenberg, P., Charu, V., Taylor, R. J., Iuliano, A. D., Bresee, J., Simonsen, L., Viboud, C., and Global Seasonal Influenza-associated Mortality Collaborator Network and GLaMOR Collaborating Teams*. 2019. “Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project.” Journal of Global Health, 9(2): 020421.
Pardi, N., Hogan, M. J., Porter, F. W., and Weissman, D. 2018. “mRNA vaccines — a new era in vaccinology.” Nature Reviews. Drug Discovery, 17(4): 261–279.
Payment, P. 2014. L’oeuvre d’Armand Frappier: 75 years of research and training to advance health and biological sciences. http://espace.inrs.ca/id/eprint/2806/
Pharmaceutical Technology. (n.d.). Novartis Vaccine Manufacturing Facility, North Carolina. Pharmaceutical Technology. Retrieved June 15, 2021, from https://www.pharmaceutical-technology.com/projects/novartis-vaccine/
Plotkin, S., Robinson, J. M., Cunningham, G., Iqbal, R., and Larsen, S. 2017. “The complexity and cost of vaccine manufacturing – An overview.” Vaccine, 35(33): 4064–4071.
Puthumana, J., Egilman, A. C., Zhang, A. D., Schwartz, J. L., and Ross, J. S. 2021. “Speed, Evidence, and Safety Characteristics of Vaccine Approvals by the US Food and Drug Administration.” JAMA Internal Medicine, 181(4): 559–560.
Quiggin, J. 2020. “Paying for what we used to own: The strange case of CSL.” Independent Australia. November 26.
Reynolds, C. 2021. “Deploy the military, Jagmeet Singh urges, to speed COVID-19 vaccinations across Canada.” National Post. February 16.
Rubin, S., and Hendrix, S. 2021. “Israel moves to head of vaccine queue, offering Pfizer access to country’s health-care database.” The Washington Post. January 28.
Rutty, C. J. (n.d.-a). Connaught Laboratories & The Making of Insulin. Connaught Fund. Retrieved October 20, 2021, from https://connaught.research.utoronto.ca/history/article3/
________. (n.d.-b). Drifting Apart: Connaught Labs & The University of Toronto, 1966-1972. Connaught Fund. Retrieved October 20, 2021, from https://connaught.research.utoronto.ca/history/article12/
________. 2020. Canada’s Vaccine Legacy: Influenza, Polio & COVID-19 Vaccine(s). The Royal Society of Canada. September 24. https://rsc-src.ca/en/voices/canada%E2%80%99s-vaccine-legacy-influenza-polio-covid-19-vaccines
Rutty, C. J., Barreto, L., Van Exan, R., and Gilchrist, S. 2005. Conquering the Crippler: Canada and the Eradication of Polio/Pour invalider la polio: Le Canada et l’eradication de la polio. Canadian Journal of Public Health; Ottawa, 96(2): I2-24.
Rynne, B., Reader, G., and Heading, S. 2020. “COVID-19 and the global economy.” KPMG. June 24. https://home.kpmg/xx/en/home/insights/2020/06/covid-19-and-the-global-economy.html
Salzer, R., Clark, J. J., Vaysburd, M., and Chang, V. T. 2021. “Single-dose immunisation with a multimerised SARS-CoV-2 receptor binding domain (RBD) induces an enhanced and protective response in mice.” BioRxiv. https://www.biorxiv.org/content/10.1101/2021.05.18.444622v1.abstract
Sanofi Canada. (n.d.). Vaccines. https://www.sanofi.ca/en/products-and-resources/vaccines
________. 2021. “Sanofi to build new manufacturing facility in Toronto to strengthen domestic pandemic preparedness and increase global supply of high-dose influenza vaccine.” http://sanoficanada.mediaroom.com/2021-03-31-
Sanofi Pasteur. 2021. Our heritage timeline. Sanofi Pasteur. March 30. https://www.sanofipasteur.co.uk/why-sanofi-pasteur/heritage-of-innovation/heritage-timeline
Scherer, F. M. 2007. “An industrial organization perspective on the influenza vaccine shortage.” Managerial and Decision Economics: MDE, 28(4–5): 393–405.
Shirley, M. M., and Walsh, P. 2001. “Public vs. Private Ownership: The Current State of the Debate.” In World Bank Policy Research Working Paper. https://papers.ssrn.com/abstract=261854
Shrotri, M., Swinnen, T., Kampmann, B., and Parker, E. P. K. 2021. “An interactive website tracking COVID-19 vaccine development.” The Lancet. Global Health. https://doi.org/10.1016/S2214-109X(21)00043-7
Strategic Advisory Group of Experts on Immunization (SAGE). (n.d.). COVID-19 vaccines technical documents. https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization/covid-19-materials
Tasker, J. P. 2021a. “Pfizer to temporarily reduce vaccine deliveries to Canada, minister says.” CBC News. January 15.
________. 2021b. “Moderna to cut deliveries to Canada in new blow to vaccination campaign.” CBC News.January 29.
________. 2021c. “Canada inks deal to produce millions of COVID-19 shots domestically.” CBC News. February 2. https://www.cbc.ca/news/politics/vaccines-canada-production-trudeau-1.5897343
________. 2021d. “Vaccine envy: Why can’t Canada make COVID-19 doses at home?” CBC News. April 28.
The Canadian Press. 2021. “More than 150,000 people in Canada experience ‘long COVID’ symptoms, report estimates.” CBC. September 15.
The World Bank. 2020. “COVID-19 Could Lead to Permanent Loss in Learning and Trillions of Dollars in Lost Earnings.” https://www.worldbank.org/en/news/press-release/2020/06/18/covid-19-could-lead-to-permanent-loss-in-learning-and-trillions-of-dollars-in-lost-earnings
Thiel, N., Selwyn, C., Murphy, G., Simpson, S., and Chakrabarti, A. C. 2021. “Recommendations for acceleration of vaccine development and emergency use filings for COVID-19 leveraging lessons from the novel oral polio vaccine.” NPJ Vaccines, 6(1):63.
Tumilty, R. 2021. “India suspends vaccine exports as cases skyrocket, stalling Canadian deliveries.” National Post. April 22.
Turnbull, S. 2021. “Feds commit nearly $200M to be able to mass produce mRNA vaccine in Canada.” CTV News. May 19.
Van Norman, G. A. 2016. “Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs.” JACC. Basic to Translational Science, 1(3): 170–179.
VIDO. (n.d.). Research. https://www.vido.org/research/
Vignier, N., and Bouchaud, O. 2018. “Travel, Migration and Emerging Infectious Diseases.” EJIFCC, 29(3): 175–179.
Walmsley, A. 1989. “Connaught’s foreign sale.” Maclean’s (Archive). December 25.
Wingrove, J. 2021. “Biden Uses Trump’s ‘America First’ Vaccine Plan to Corner Market.” Bloomberg. March 24.
Wolfe, N. D., Dunavan, C. P., and Diamond, J. 2007. “Origins of major human infectious diseases.” Nature 447, (7142): 279–283). https://doi.org/10.1038/nature05775
Wolfson, M., and Knoppers, B. M. 2021, December 8). “Canadians’ health data are in a shambles.” The Globe and Mail. December 8. https://www.theglobeandmail.com/opinion/article-canadians-health-data-are-in-a-shambles/
Xiao, X., Newman, C., Buesching, C. D., Macdonald, D. W., and Zhou, Z.-M. 2021. “Animal sales from Wuhan wet markets immediately prior to the COVID-19 pandemic.” Scientific Reports, 11(1): 11898.
Yarr, K. 2021. “P.E.I. company to produce mRNA vaccines.” CBC News. November 18.









